

Which one of the following elements found a place in the periodic table later?GermaniumChlorineOxygenSilicon A property closely related to an atom’s mass number is its atomic mass.
In Mendeleev’s periodic table, gaps were left for the elements to be discovered later. How does the number of valence electrons vary on moving from left to right:(i) In the first period of the periodic table? (ii) In the second period of the periodic table?. Consider the following elements:Na, Ca, Al, K, Mg, Li (a) Which of these elements belong to the same period of the periodic table? (b) Which of these elements belong to the same group of the periodic table?. (a) What is the main characteristic of the last elements in the periods of the periodic table? What is the general name of such elements?(b) What is the number of elements in: (a) 1st period, and (b) 3rd period, of the modern periodic table?. 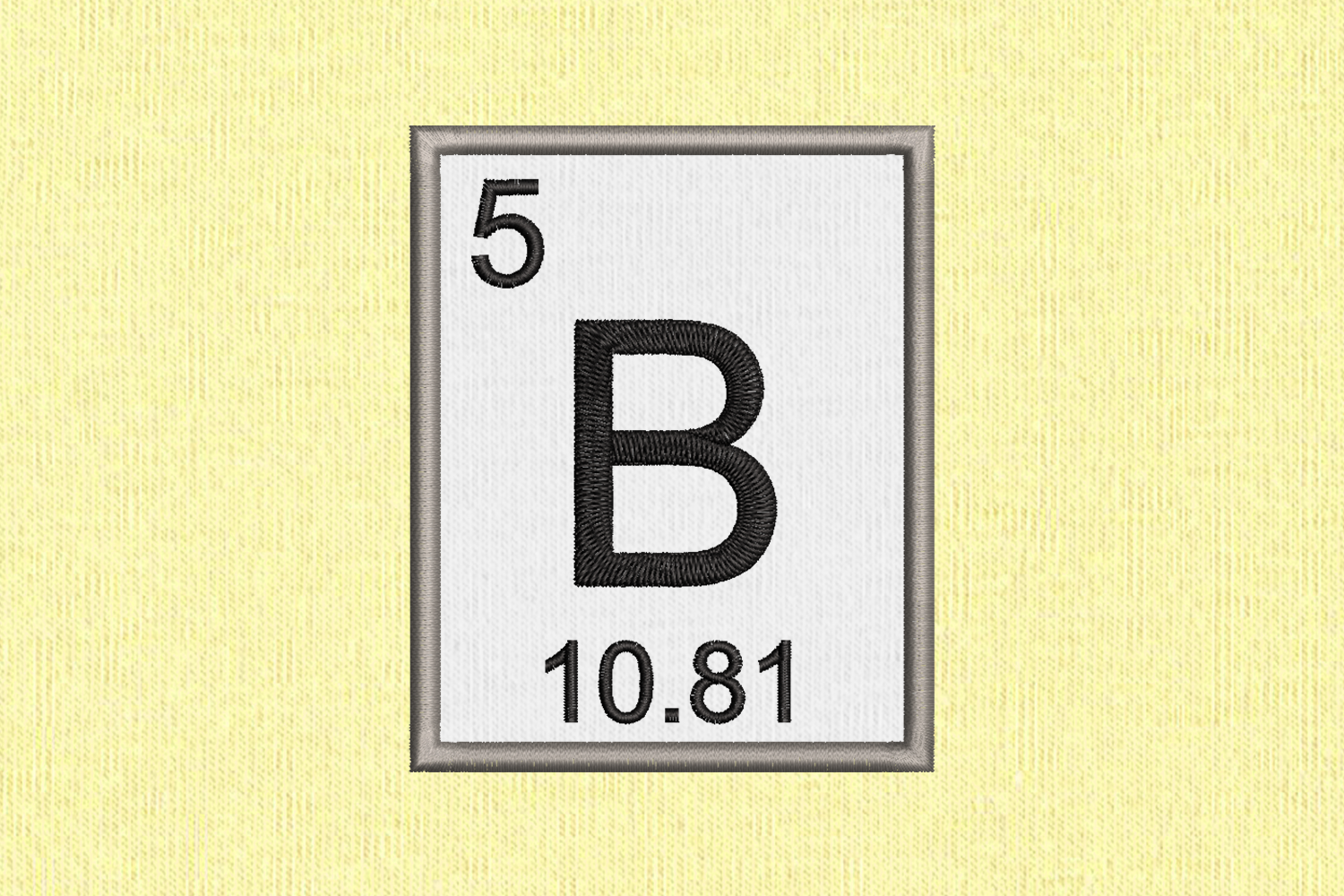 Define the Periodic Table with elements name. Atomic number of a few elements are given below 10, 20, 7, 14(a) Identify the elements(b) Identify the Group number of these elements in the Periodic Table(c) Identify the Periods of these elements in the Periodic Table(d) What would be the electronic configuration for each of these elements?(e) Determine the valency of these elements. (a) What is the usual number of valence electrons and valency of group 18 elements of the periodic table?(b) What happens to the number of valence electrons in the atoms of elements as we go down in a group of the periodic table?. (a) How does the electropositive character of elements change on going down in a group of the periodic table?(b) State how the valency of elements varies (i) in a group, and (ii) in a period, of the periodic table. Virtually all elements exist as mixtures of isotopes, so atomic masses may vary significantly from whole numbers. In the modern periodic table, which are the metals among the first ten elements? The atomic mass of boron is calculated similarly to what we did for our hypothetical example, but the percentages are different: Thus, we use 10.8 u for the atomic mass of boron. Compare and contrast the arrangement of elements in Mendeleev’s Periodic Table and the Modern Periodic Table. What is the major characteristic of the first elements in the periods of the periodic table? What is the general name of such elements?. Name of 118 elements in the periodic table. How does the valency of elements change on moving from left to right in the third period of the periodic table?. How does the valency of elements vary in going down a group of the periodic table?. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Appearance: Yellow-brown non-metallic crystal Element Boron (B), Group 13, Atomic Number 5, p-block, Mass 10.81. Electrochemical equivalence: 0.1344 g/amp-hr. Pure boron was first isolated by the French chemists, J.L. Optically and visually, crystallized boron exhibits structures like diamonds and it is almost equally hard.
Define the Periodic Table with elements name. Atomic number of a few elements are given below 10, 20, 7, 14(a) Identify the elements(b) Identify the Group number of these elements in the Periodic Table(c) Identify the Periods of these elements in the Periodic Table(d) What would be the electronic configuration for each of these elements?(e) Determine the valency of these elements. (a) What is the usual number of valence electrons and valency of group 18 elements of the periodic table?(b) What happens to the number of valence electrons in the atoms of elements as we go down in a group of the periodic table?. (a) How does the electropositive character of elements change on going down in a group of the periodic table?(b) State how the valency of elements varies (i) in a group, and (ii) in a period, of the periodic table. Virtually all elements exist as mixtures of isotopes, so atomic masses may vary significantly from whole numbers. In the modern periodic table, which are the metals among the first ten elements? The atomic mass of boron is calculated similarly to what we did for our hypothetical example, but the percentages are different: Thus, we use 10.8 u for the atomic mass of boron. Compare and contrast the arrangement of elements in Mendeleev’s Periodic Table and the Modern Periodic Table. What is the major characteristic of the first elements in the periods of the periodic table? What is the general name of such elements?. Name of 118 elements in the periodic table. How does the valency of elements change on moving from left to right in the third period of the periodic table?. How does the valency of elements vary in going down a group of the periodic table?. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images. Appearance: Yellow-brown non-metallic crystal Element Boron (B), Group 13, Atomic Number 5, p-block, Mass 10.81. Electrochemical equivalence: 0.1344 g/amp-hr. Pure boron was first isolated by the French chemists, J.L. Optically and visually, crystallized boron exhibits structures like diamonds and it is almost equally hard. 
Pure boron, on the other hand, is an electrical conductor just like carbon. When forming other compounds, boron acts like a non-metallic compound. Thus, many boron compounds can be used in different industries.

It forms compounds with various metallic and non-metallic elements of different characteristics. Note that each element may contain more isotopes. For the B10 isotope, the natural rate of occurrence is 19.1–20.3%, whereas for the B11 it is 79.7–80.9%.Ĭhemically unbound boron cannot be found naturally on Earth. Boron occurs naturally in the form of two stable isotopes, namely B10 and B11. Located in the 3A group of the periodic table, boron is a semiconductor metalloid. Boron is an element on the periodic table with symbol B, atomic number 5, and atomic weight 10.81.








 0 kommentar(er)
0 kommentar(er)
